A clinical study of T-regulatory lymphocyte function in cancer patients in relation to tumor histotype, disease extention, lymphocyte subtypes and cortisol secretion
Research Article (download PDF version)
Luigi Vigorè1, Fernando Brivio2, Luca Fumagalli2, Roberto Vezzo1, Giusy Messina6, Franco Rovelli6, Massimo Colciago3, Giovanna D’Amico4, Giuseppe Di Fede5, Paolo Lissoni6
1 Laboratory of Immunomicrobiology, St.Gerardo Hospital, Monza, Milan, Italy
2 Division of Surgery, St.Gerardo Hospital, Monza, Milan, Italy
3 I.N.R.C.A Laboratory of Analyses, Casatenovo, Lecco, Italy
4 Research Center “Fondazione Tettamanti” Clinica Pediatrica Università Milano-Bicocca, Italy
5 Institute of Biological Medicine, Milan, Italy
6 Division of Radiation Oncology, St.Gerardo Hospital, Monza, Milan, Italy
*Correspondence: Dr. Paolo Lissoni, Divisione di Radioterapia Oncologica, Ospedale S.Gerardo, 20052 Monza, Milano, Italy; Fax: +390392332284, E-mail: p.lissoni@hsgerardo.org
Key words: Anticancer immunity, immunosuppression, T regulatory lymphocytes
Abbreviations: cytotoxic T lymphocyte-associated antigen-4, (CTLA-4); glucocorticoid-induced TNF-α receptor, (GITR); myeloidderived suppressor cells, (MDSC); NK cells, (CD16CD56); T cytotoxic lymphocytes, (CD8); T helper lymphocytes, (CD4); Tregulatory lymphocytes, (T-reg)
Received: 24 July 2008; Revised: 11 September 2008
Accepted: 12 September 2008; electronically published: October 2008
Summary
Several clinical investigations showed that the immune status is a prognostic variable in cancer patients, even tough the evaluation of the anticancer immunity is not generally considered in the medical oncology. Several immune parameters, including lymphocyte subsets and cytokine blood concentration, had been proposed to quantify the functional status of the anticancer immunity, but recent discoveries would suggest that the end-result of the various immune interactions is represented by a subtype of CD4 lymphocytes capable of suppressing the antitumor immune reaction, the so called T-regulatory lymphocytes (T-reg). This study was performed to detect T-reg count and percentage in solid tumor patients, in relation to tumor histotype, disease extension, lymphocyte sub-populations and cortisol circadian secretion. The study included 114 consecutive cancer patients affected by the most frequent tumor histotypes, 69 of whom showed a metastatic disease. In each patient we evaluated T-reg cells, identified as CD4+CD25+, in relation to T helper (CD4), T cytotoxic (CD8) and NK (CD16CD56) cells. Abnormally high values of T-reg cells were seen in 52/114 (46%) patients, and the percentage of high values of T-reg was significantly higher in metastatic patients than in non-metastatic ones. In contrast, no significant difference was seen in relation to tumor histotype. Patients with increased T-reg count had a significantly lower NK cell number. Finally no significant difference in T-reg number was seen between patients with altered or normal rithm of cortisol. The study confirmed that, irrespectively of tumor histotype the metastatic disease is associated with a progressive and increased T-reg generation, with a following suppression of anticancer immunity.
I. Introduction
At present, there is no doubt about the existence of a sub-type of T lymphocytes, the so-called T regulatory lymphocytes (T-reg), capable of suppressing the cellular immune responses,including the anticancer immunity (Thomton and Shevach, 2000; Shevach, 2002; von Herrath and Harrison, 2003; Schwartz, 2005; von Boehmer, 2005; Ziegler, 2006; Zou, 2006). However, the exact definition
of T-reg cells in terms of cell surface marker expression still remains controversial, particularly from a clinical point of view. All authors are in agreement to consider Treg lymphocytes as CD4+CD25+ cells, but at present it is still unknown whether the expression of CD4 and CD25 antigens may be sufficient to identify T-reg cells (Thomton and Shevach, 2000; Shevach, 2002; von Herrath and Harrison, 2003; Schwartz, 2005; von Boehmer, 2005; Ziegler, 2006; Zou, 2006), since several authors retain that the intracytoplasmatic expression of the FOX p3 protein is essential for the differentiation into T-reg cells (Ziegler, 2006; Zou, 2006).Recently, however, some preliminary observations would suggest that the cytoplasmatic expression of FOX p3 by CD4+CD25+ cells may be associated at least in some experimental conditions with a diminished, rather than with an enhanced immunosuppressive activity of T-reg cells (Siddiqui et al, 2007). In contrast, all authors agree that the expression of CD152 antigen, also called cytotoxic T lymphocyteassociated antigen-4 (CTLA-4) (Vasu et al, 2004), is fundamental for the immunosuppressive activity of T-reg cells (Takahashi et al, 2000), since the block of its expression by using anti-CTLA-4 monoclonal antibodies may abolish the suppressive activity of T-reg cells, with a following stimulation of the anticancer immunity in cancer patients (Knutson and Disis, 2007) and an enhanced incidence of autoimmune diseases in the healthy subjects (Lan et al, 2005). Therefore, the addition of a third marker, such as CD152 antigen, may allow to define a more homogeneous cell population provided by a regulatory activity with respect to the simple CD4+CD25+ expression (Dieckmann and Plottner, 2001). In fact, the suppressive regulatory action of CD4+CD25+CD152+ has appeared to be clearly higher than that played by the simple CD4+CD25+ T lymphocytes (Leong et al, 2006).This finding is not surprising, since the simple expression of CD25 marker, corresponding to the !-chain of IL-2 receptor, is not an exclusive characteristic of T-reg lymphocytes, but it is a non-specific property of the overall activated T lymphocytes (Thomton and Shevach, 2000; Shevach, 2002; von Herrath and Harrison, 2003; Schwartz, 2005; von Boehmer, 2005; Ziegler, 2006; Zou, 2006). At present, preliminary clinical studies would show that the percent of circulating CD4+CD25+ cells may be about 10% of the all CD4+ lymphocytes, and that of CD4+CD25+CD152+ cells may be about 40% of the total CD4+CD25+ cells, then the expected percent of CD4+CD25+CD152+ in the healthy subjects would be less than 5% of the total circulating CD4+ lymphocytes (Jago et al, 2004). Finally, the expression of glucocorticoidinduced TNF-α receptor (GITR) is also associated with an evident suppressive activity by T-reg lymphocytes (Kanamaru et al, 2004), which in fact are stimulated by
cortisol (Sthephens et al, 2004), that in contrast may inhibit the activity of the most other T lymphocytes, namely that of T helper lymphocytes, with a following diminished production of IL-2 (Claman, 1998). As far as the mechanisms responsible for T-reg-induced suppression of the anticancer immunity are concerned, several experimental observations have shown that T-reg cells may suppress the antitumor immune response through the release of immunosuppressive cytokines, namely IL-10 and TGF-β (Dieckmann et al, 2002), even though other authors would suggest that the suppressive activity of Treg cells on CD4+ and CD8+ lymphocyte activation may be relatively independent from the action of cytokines, by mainly requiring cell surface contact (Birebent et al, 2004). IL-2 has been proven to be essential for T-reg generation and some authors consider IL-2 as the main growth factor of T-reg lymphocytes (Antony and Restito, 2005), but more adequate studies have demonstrated that IL-2 may induce both stimulation and inhibition of T-reg generation and activation (Malek and Bayer, 2004). In fact, IL-2 has appeared to induce and promote T-reg differentiation only in the presence of TGF-β (Chen et al, 2003). Therefore, IL-2 would constitute the main human cytokine in influencing the characteristics of the anticancer immunity, since it may be responsible for both activation and suppression of an effective immune response against cancer cell proliferation and dissemination (Wang et al, 2001), namely depending on the whole status of the cytokine network, in particular on the presence or in the absence of adequate concentrations of TGF-β. In the absence of TGF-β, IL-2 stimulates the anticancer immunity, whereas it counteracts the generation of an effective antitumor immunity in the presence of TGF-β. In other words, IL-2 would physiologically control both tolerance and immunity, depending on the presence of TGF-β and other less known factors (Annunziato et al, 2002). In fact, under cancer immunotherapy with IL-2 the percent of T-reg cells has been shown to decrease in responding patients and to enhance in those with disease progression (Cesana et al, 2006). However, the regulation of T-reg functions does not depend only on immune factors, since it is also under a neuroendocrine control (Ji et al, 2004). In particular, cortisol has appeared to stimulate T-reg cell generation (Ji et al, 2004), with a following enhanced release of IL-10, by representing the main mechanism responsible for cortisol-induced immunosuppression. From a clinical oncological point of view, preliminary observations showed an enhanced percent of circulating CD4+CD25+ lymphocytes in cancer patients, namely in those with advanced disease (Sasada et al, 2003). The present study was performed to better establish which is T-reg behaviour in cancer patients in relation to both tumor histotype and disease extension.
II. Materials and methods
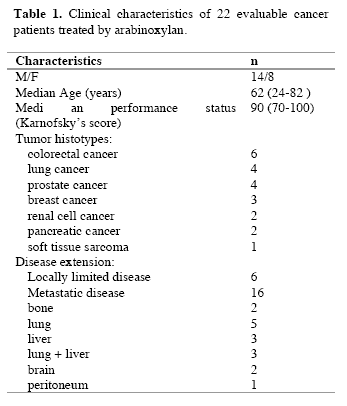
The study included 114 consecutive solid tumor patients with locally limited or metastatic disease, whose clinical characteristics are shown in Table 1. Lung cancer and gastrointestinal tumors were the most frequent neoplasms in our patients. For the immune detections, venous blood samples were collected in the morning after an overnight fast. Operable patients and metastatic patients were investigated before the surgical operation and before the onset of chemotherapy, respectively, in an attempt to exclude the possible influence of the various anticancer therapies on the immune status of patients.
In each sample, we have evaluated total lymphocyte count and the various lymphocyte subpopulations by a flow cytometric assay and monoclonal antibodies, including T helper lymphocytes (CD4), T cytotoxic lymphocytes (CD8), NK cells (CD16CD56), and T regulatory (T-reg) lymphocytes (CD4CD25). Normal values (95% confidence limits) of T-reg observed in our laboratory were below 240/mm3. Moreover, because of its importance in regulating lymphocyte functions and proliferation (Claman, 1998; Sthephens et al, 2004), the circadian rhythm of cortisol was also investigated by collecting blood samples at 8.00 A.M. and at 4.00 P.M., and cortisol serum concentrations were measured in duplicate by using the ECLA method. Data were reported as mean +/- SE, and statistically analyzed by the Student’s t test, the analysis of variance and the chi-square test, as appropriate.
III. Results
As reported in Table 2, an abnormally high number of T-reg was seen in 52/114 (46%) patients. Moreover, the percentage of cases with elevated number of T-reg observed in metastatic patients was significantly higher with respect to that found in non-metastatic patients (44/69 (64%) vs 8/45(18%), p < 0.01). Table 3 shows the mean number of T-reg and the mean percentages of T-reg with respect to both total lymphocytes and T helper (CD4+) lymphocytes observed in cancer patients in relation to their disease extension. The mean number of T-reg observed in metastatic patients was higher with respect to that found in patients with locally limited disease, without, however statistically significant differences. In contrast, the mean percentages of T-reg with respect to that of both lymphocytes and CD4 cells were significantly higher in metastatic patients than in the non-metastatic ones (p< 0.05 and p< 0.001,respectively). Moreover, within the metastatic group, patients with a normal lymphocyte count greater than 1500/mm3 showed a significantly higher mean number of T-reg with respect to the non-metastatic patients, whereas no difference was seen between nonmetastatic patients and metastatic patients with lymphocytopenia, consisting of lymphocyte count lower than 1500/mm3. In contrast, the mean percentages of T-reg with respect to total lymphocytes and CD4+ cells observed in both groups of metastatic patients with normal or low total lymphocyte count were significantly higher than in non-metastatic patients (lymphocytes: p< 0.025, CD4+ cells: p< 0.001).
Table 1. Clinical characteristics of 114 solid tumor patients
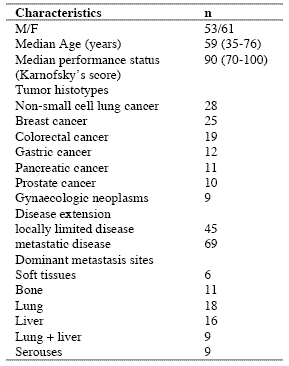
Table 2. Percentages of abnormally high values of CD4+CD25+ lymphocytes
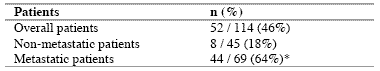
* P < 0.01 vs non-metastatic patients
Table 3. Mean number of CD4+CD25+ lymphocytes and their mean percentages with respect to total lymphocytes and CD4+ lymphocytes in metastatic and non-metastatic patients
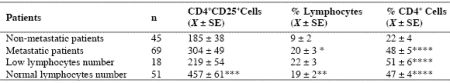
* p<0.05 vs non-metastatic patients; ** p<0.025 vs non-metastatic patients; *** p<0.01 vs non-metastatic patients; **** p<0.001 vs nonmetastatic patients
The mean counts of NK and CD8 cells in relation to that of T-reg are reported in Table 4. As shown, no significant difference in the mean number of CD8 lymphocytes was found between patients with normal or abnormally elevated number of T-reg. On the contrary, patients with elevated number of T-reg showed a significantly lower number of NK cells with respect to that found in those with normal T-reg count. Finally, Table 5 shows the circadian rhythm of cortisol in relation to total lymphocytes, CD4+ cells and T-reg mean number. A normal cortisol rhythm, with morning values greater at least than 50% with respect to the values occurring during the afternoon, was found in 85/114 (75%). Total lymphocyte and CD4+ cell mean numbers observed in patients with altered cortisol rhythm were significantly lower than those found in patients with normal cortisol circadianicity (p<0.01), whereas no significant difference was seen in the mean number of T reg. Figure 1 and Figure 2 illustrate T-reg mean numbers in relation to tumor histoptypes in the overall patients and with respect to their disease extension, respectively. No significant difference was seen in relation to tumor histotype. The highest values of T-reg were observed in pancreatic cancer patients, without however significant differences with respect to the overall other histotypes. The metastatic disease was associated with a higher number of T-reg with respect to the non-metastatic group in all tumor histotypes, even though a statistically significant differences occurred for the only breast cancer (p<0.05) and colorectal cancer (p< 0.01).
Table 4. Mean values of NK cells and CD8+ lymphocytes in cancer patients with normal or abnormally high values of CD4+CD25+ lymphocytes

* p<0.05 vs normal values of CD4+CD25+ lymphocytes
Table 5. Mean numbers of total lymphocytes, T helper (CD4+) lymphocytes and T regulator lymphocytes (CD4+CD25+) in relation to cortisol circadian secretion in cancer patients

* P<0.01 vs patients with altered cortisol rhythm
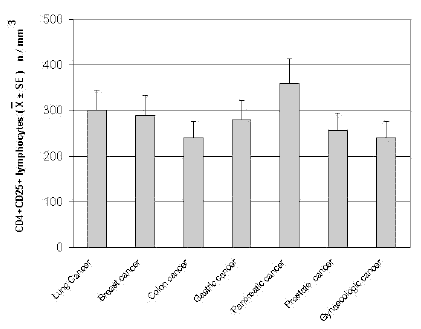
Figure 1. CD4+CD25+ lymphocyte mean number in relation to tumor histotype
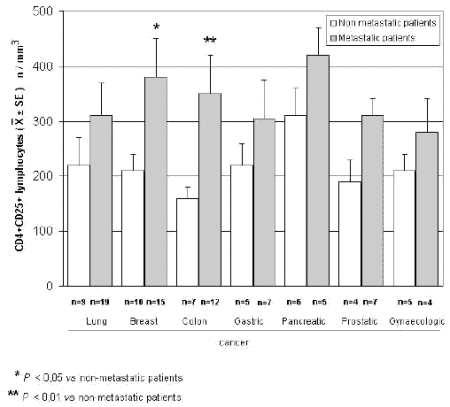
Figure 2. CD4+CD25+ lymphocytes in relation to tumor histotype in metastatic and non-metastatic cancer patients
IV. Discussion
According to previous preliminary clinical investigations (Sasada et al, 2003; Cesana et al, 2006), this study confirms in a greater number of cancer patients that the metastatic disease is characterized by the evidence of an abnormally increased percentage of T-reg lymphocytes with respect to both total circulating lymphocytes and CD4+ lymphocytes. This finding does not seem to represent a specific characteristic of some tumor histotypes, then it could constitute a general alteration occurring during the progression of the neoplastic disease, by representing a fundamental immune parameter of cancer-related immunosuppression.
Several immune molecules have appeared to suppress the anticancer immunity, namely IL-6, IL-10, IL-1, TNF-α and TGF-β, but it seems that the common end result of their mechanisms of action may be represented by the stimulation of T-reg generation, with a consequent inhibition of the activation of an effective anticancer immune reaction. On the same way, several immune cells are able to suppress the anticancer immunity, including macrophages, T helper-2 lymphocytes and some myeloidderived suppressor cells, but also in this case they would act in a suppressive way by promoting the generation of Treg.
Then, the detection of T-reg amounts in terms of both absolute number and percentages with respect to total lymphocytes and CD4+ cells could constitute a simple and adequate clinical immune parameter to quantify the whole status of the anticancer immunity in the single cancer patient. Moreover, future clinical studies will be required to establish the possible prognostic significance of changes in T-reg percentage and number in relation to the anticancer efficacy of the various standard antitumor therapies. Moreover, it has to be remarked that T-reg lymphocytes would not represent the only immune cells involved in the suppression of the anticancer immunity. In fact, there is at least another fundamental immunosuppressive system, consisting of the monocytemacrophage cell lineage (Sica and Bronte, 2007). In more detail, it has been observed that the bone marrow may release myeloid precursors provided by suppressive activity on the antitumor immune response and defined as myeloid-derived suppressor cells (MDSC) (Kusmartsev and Gabrilovich, 2005). These cells have appeared to be characterized by the cell surface expression of GR-1, CD11b and CD80 antigens (Anderson et al, 2002; van Ginderachter et al, 2006). The myeloid suppressor cells would promote the generation and activation of T-reg lymphocytes, which at the other side would stimulate MDSC release from the bone marrow and M2 macrophage differentiation (Terabe et al, 2003; Wie et al, 2006).
Moreover, the myeloid suppressive cells would inhibit the anticancer immunity by promoting macrophage differentiation into the M2 sub-type (Ikemoto et al, 2003), which plays a clear inhibitory effect on the anticancer immunity, namely through the release of IL-6 (Ueno et al, 2000), whereas the M1 macrophage sub-type may either stimulate or suppress the antitumor immunity (Mantovani et al, 2004). M1 and M2 macrophage sub-types have appeared to be characterized by a high production of IL-12 or IL-10, respectively (Ueno et al, 2000).
Then, further studies by concomitantly evaluating T reg and MDSC count, will contribute to better define the immune mechanism responsible for the suppression of the anticancer immunity.
References
Anderson CF, Gerber JS, Mosser DM (2002) Modulating macrophage function with IgG immune complexes. J Endotoxin Res 8, 477-81.
Annunziato F, Cosmi L, Liotta F, Lazzeri E, Manetti R, Vanini V, Romagnani P, Maggi E, Romagnani S (2002) Phenotype,localization and mechanism of suppression of CD4+CD25+ human thymocytes. J Exp Med 196, 379-87.
Antony PA, Restito NP (2005) CD4+CD25+ T regulatory cells,immunotherapy of cancer,and interleukin-2. J Immunother 28, 120-8.
Birebent B, Lorho R, Lechartier H, de Guibert S, Alizadeh M,
Vu N, Beauplet A, Robillard N, Semana G (2004) Suppressive properties of human CD4+CD25+regulatory T cells are dependent on CTLA-4 expression. Eur J Immunol 34, 3485-96.
Cesana GC, DeRaffele G, Cohen S, Moroziewicz D, Mitcham J, Stoutenburg J, Cheung K, Hesdorffer C, Kim-Schulze S, Kaufman HL (2006) Characterization of CD4+CD25+ regulatory T cells in patients treated with high-dose interkleukin-2 for metastatic melanoma or renal cell carcinoma. J Clin Oncol 24, 1169-77.
Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM (2003) Conversion of peripheral CD4+CD25- naïve T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp 3. J Exp Med 198, 1875-86.
Claman HN (1998) Corticosteroids and the immune system. Adv Exp Med Biol 245, 203-10.
Dieckmann D, Bruett H, Ploettner H, Lutz MB, Schuler G (2002) Human CD4+CD25+ regulatory contact-dependent T cell induce IL-10 producing,contact-independent type-1-regulatory T cells. J Exp Med 196, 247-53.
Dieckmann D, Plottner H (2001) Ex vivo isolation and characterizationof CD4+CD25+ T cells with regulatory properties from human blood. J Exp Med 193, 1303-10.
Ehrke MJ, Mihich E, Berd D, Mastrangelo MJ (1989) Effects of anticancer drugs on the immune system. Semin Oncol 16, 230-9.
Ghiringhelli F, Larmonier N, Schmitt E, Parcellier A, Cathelin D, Garrido C, Chauffert B, Solary E, Bonnotte B, Martin F (2004) CD4+ CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows Immunotherapy of established tumors to be curative. Eur J Immunol 34, 336-44.
Ikemoto S, Yoshida N, Narita K, Wada S, Kishimoto T, Sugimura K, Nakatani T (2003) Role of tumor-associated macrophages in renal cell carcinoma. Oncol Rep 10, 1843-9.
Jago CB, Yates J, Camara NOS, Lechler RI, Lombardi AG (2004) Differential expression of CTLA-4 among T cell subsets. Clin Exp Immunol 136, 463-71.
Ji HB, Liao G, Faubion WA, Abadía-Molina AC, Cozzo C, Laroux FS, Caton A, Terhorst C (2004) Cutting edge, the natural ligand for glucocorticoid-induced TNF receptorrelated protein abrogates regulatory T cell suppression. J Immunol 172, 5823-7.
Kanamaru F, Youngnak P, Hashiguchi M, Nishioka T, Takahashi T, Sakaguchi S, Ishikawa I, Azuma M (2004) Costimulation via glucocorticoid-induced TNF receptor in both conventional and CD25+ regulatory CD4+ T cells. Immunol 172, 7306-14.
Knutson KL, Disis M, Salazar L (2007) CD4 regulatory T cells in human cancer pathogenesis. Cancer Immunol Immunother 556, 271-85.
Kusmartsev S, Gabrilovich DI (2005) STAT1 signaling regulates tumor-assolciated macrophage-mediate T cell deletion. J Immunol 174, 4880-91.
Lan RY, Ansari AA, Lian ZX, Gershwin ME (2005) Regulatory T cells, development,function,and role in autoimmunity. Autoimmun Rev 4, 351-63.
Leong PP, Mohammad R, Ibrahim N, Ithnin H, Abdullah M, Davis WC, Seow HF (2006) Phenotyping of lymphocytes expressing regulatory and effector markers in infiltrating ductal carcinoma of the breast. Immunol Lett 102, 229-36.
Malek TR, Bayer AL (2004) Tolerance not immunity crucially depends on IL-2. Nat Rev Immunol 4, 665-74.
Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M (2004) The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 25, 677-86.
Sasada T, Kimura M, Yoshida Y, Kanai M, Takabayashi A (2003) CD4+CD25+ regulatory T cells in patients with gastrointestinal malignancies, possible involvement of regulatory T cells in disease progression. Cancer 98, 1089-93.
Schwartz RH (2005) Natural regulatory T cells and selftolerance. Nat Immunol 6, 327-30.
Shevach EM (2002) CD4+CD25+ suppressor T cells, more questions than answers. Nat Rev Immunol 2, 389-400.
Sica A, Bronte V (2007) Altered macrophage differentiation and immune dysfunction in tumor development. J Clin Invest 117, 1155-66.
Siddiqui SA, Frigola X, Bonne-Annee S, et al (2007) Tumorinfiltrating Foxp3 CD4+CD25+ T cells predict poor survuival in renal cell carcinoma. Clin Cancer Res 13, 2075-81.
Stephens GL, McHugh RS, Whitters MJ, Young DA, Luxenberg D, Carreno BM, Collins M, Shevach EM (2004) Engagement of glucocorticoid-induced TNFR family-related receptor on effector T cells by its ligand mediates resistance to suppression by CD4+CD25+ T cells. J Immunol 173, 5008-20.
Takahashi T, Tagami T, Yamazaki S, Uede T, Shimizu J, Sakaguchi N, Mak TW, Sakaguchi S (2000) Immunologic and tolerance maintained by CD25+CD4+ regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen. J Exp Med 192, 1285-94.
Terabe M, Matsui S, Park JM, Mamura M, Noben-Trauth N, Donaldson DD, Chen W, Wahl SM, Ledbetter S, Pratt B, Letterio JJ, Paul WE, Berzofsky JA (2003) Transforming growth factor-β production and myeloid cells are an eggector mechanism trhough which CD1d-restricted T cells block cytotoxic T lymphocyte-mediated tumor immunosurveillance, abrogation prevents tumor recurrence. J Exp Med 198, 1741-52.
Thomton AM, Shevach EM (2000) Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J Immunol 164, 183-90.
Ueno T, Toi M, Saji H, Muta M, Bando H, Kuroi K, Koike M, Inadera H, Matsushima K (2000) Significance of macrophage chemo-attractant protein-1 in macrophage recruitment,angiogenesis and survival in human breast cancer. Clin Cancer Res 6, 3282-9.
Van Ginderachter JA, Movahedi K, Hassanzadeh Ghassabeh G, Meerschaut S, Beschin A, Raes G, De Baetselier P (2006) Classical and alternative activation of mononuclear phagocytes, picking the best of both worlds for tumor promotion. Immunobiology 211, 487-501.
Vasu C, Prabhakar BS, Holterman MJ (2004) Targeted CTLA-4 engagement induces CD4+CD25+CTLA-4 high T regulatory cells with target alloantigens specificity. J Immunol 173, 2866-76.
von Boehmer H (2005) Mechanisms of suppression by suppressor T cells. Nat Immunol 6, 338-44.
von Herrath MG, Harrison LC (2003) Regulatory lymphocytes, antigen-induced regulatory T cells in autoimmunity. Nat Rev Immunol 3, 223-32.
Wang XB, Zheng CY, Giscombe R, Lefvert AK (2001) Regulation of surface and intracellularexpression of CTLA-4 on human peripheral T cells. Scand J Immunol 54, 453-8.
Wie S, Kryczeck I, Zou W (2006) Regulatory T-cell compartmentalization and trafficking. Blood 108, 426-31.
Yang R, Cai Z, Zhang Y, Yutzy WH 4th, Roby KF, Roden RB (2006) CD80 in immune suppression by mouse ovarian carcinoma-associated Gr-1+CD11b+ myeloid cells. Cancer Res 66, 6807-15.
Ziegler SF (2006) FOXP3 of mice and men. Annu Rev Immunol 24, 209-26.
Zou W (2006) Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol 6, 295-307.
(download PDF version)